
|
Click here to visit Richard S. Levine's entire Lithium Bromide website with more articles, features and technical information. |
PLANT ENGINEERING, October 12, 1978
Lithium bromide is an essential ingredient in the operation of absorption chillers; however,
it is a very corrosive salt capable of destroying various metals in the machine.
Here is some helpful information for
Avoiding Problems from Lithium Bromide in Absorption Chillers
By RICHARD S. LEVINE
ABSORPTION REFRIGERATION is an excellent central air-conditioning method for plants that have excess boiler capacity to provide the steam needed to drive the chiller. Absorption chillers can deliver from 25 to 1200 tons of refrigeration.
The absorption system shown in the illustration uses water as the refrigerant. The water is flash evaporated in a vacuum to provide the necessary change in enthalpy for cooling a chilled-water loop. Chilled water entering the absorber at ambient (or lower) temperatures is optimally cooled to 44 F.
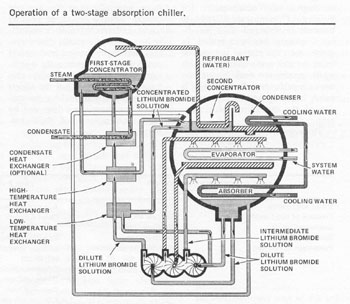
If water vapor accumulates to the point at which the vacuum is diminished, the cycle will stop. Therefore, lithium bromide is added to absorb the water vapor and perpetuate the cycle.
Lithium bromide is a salt and desiccant (drying agent). The lithium ion (Li+) in the lithium bromide solution and the water molecules have a strong association, producing the absorption essential for the chiller to operate.
Lithium bromide concentrations between 58 and 62 percent are used in absorption
chillers. This range provides maximum efficiency for absorption of the flash-evaporated water. The diluted brine is then boiled to remove the excess water. The brine is returned to the system for additional absorption, the water is released for subsequent evaporation, and the cycle is completed.
This brief explanation is obviously an oversimplification. A rather involved mechanical system is required for these actions. For example, heat exchangers optimize the energy transfer between the hot, concentrated lithium bromide that is recycling and the cooler, dilute brine that is yet to be boiled. Spray headers facilitate the flash evaporation and absorption process. Pumps move the various solutions from one point to another, and the customary assortment of valves, switches, and tube bundles are required. (See PE 3/2/78, p 65, and 9/15/77, p 169, file #2510, for a thorough discussion of absorption chillers.)
One important yet often overlooked point is that lithium bromide is very corrosive and can destroy various metals used in the absorption chiller. The relationship between lithium bromide and absorption chillers will be discussed in this three-part series. This first article analyzes the lithium bromide solution, the second will discuss corrosion and inhibitors, and the third will explain procedures for cleaning the accumulated corrosion debris from the machine.
Two Factors—Before the chemical analysis of lithium bromide can be discussed and the results interpreted, two factors must be pointed out.
First, the chemistry of the brine is important, but other factors also influence the proper operation and maintenance of a steam-absorption unit. Good mechanical procedures must always be followed. Chemical adjustment and corrosion control are essential to prevent deterioration and failure of the equipment, but they are not a substitute for proper maintenance by qualified personnel. The chemical methods supplement the mechanical ones.
Second, the key point concerning the operation and maintenance of the steam absorber is that the water is flash evaporated in a vacuum. If the vacuum is maintained and air cannot leak into the chiller, corrosion will be considerably reduced or eliminated.
Chemical Analysis—An analysis is required to evaluate the chemical condition of the lithium bromide. The sample must be representative of the majority of the machine’s solution for the condition of the lithium bromide charge to be evaluated properly.
Samples can be taken at various points in the machine. Dilute lithium bromide is found after the charge has absorbed water and is moving to the concentrator for boiling. Concentrated lithium bromide is obtained after the diluted material has been boiled. In some cases, the charge is removed from the unit and stored in barrels while repairs are made or maintenance is performed. Each sample is valid, but may not be representative of the overall chemical content.
The representative samples provided to the laboratory for analysis and interpretation may be taken from an operating unit under normal load conditions. The best test locations and the correct procedure for drawing samples should be obtained from the manufacturer.
When samples cannot be taken under a load situation, the lithium bromide can still be accurately monitored by mixing samples from several points in the absorption machine.
Lithium bromide chemistry is a non-equilibrium process and reactions constantly occur, even in the sample container. In as little as 24 hours, brine chemistry can change significantly, particularly if there are external influences such as air leaks. It is critical that the laboratory receive and evaluate samples quickly. An experienced interpreter will recognize the time factor involved and, using prior experience, will be able to adjust for changes.
Table I lists the 10 tests normally conducted on lithium bromide solutions. The methods of analysis are similar to those employed for routine chemical testing, but there are some distinct differences.
TABLE I. Tests Required For Lithium Bromide Analysis
|
Visual Appearance |
|
Presence of Octyl Alcohol |
|
Suspended Solids |
|
Specific Gravity |
|
Percentage of Solution |
|
pH |
|
Solution Alkalinity |
|
Dissolved Copper |
|
Dissolved Iron |
|
Inhibitor Concentration |
Most important, tests for lithium bromide must he more precise than those normally used for a water sample from a boiler or cooling tower. Lithium bromide is normally in a relatively small, closed system, and slight changes in chemical composition are extremely significant. Cooling towers or boilers also have water changes, but the effects are greatly diminished by the system’s chemistry over time and by the vast amounts of fluid employed.
In addition, many specific corrosion tests are performed on the lithium bromide solution, but are not reported to the plant engineer because their values are limited. The analysis is not designed to turn the plant engineer into a chemist. Instead, the analysis should explain the condition of the absorber without burdening him with technical data that would have meaning only to trained personnel.
Types of Tests—The first three tests are qualitative and indicate the physical condition of the charge.
Normally, new lithium bromide solutions are clear and colorless. Visual appearance usually reveals contamination such as dirt and debris, which is mostly iron oxide.
In normal absorber operation, octyl alcohol is added to the charge as a wetting agent and defoamer. The chemical maximizes contact between the lithium bromide and the tubes for the greatest heat exchange and reduces foaming of the lithium bromide as vacuum is applied.
Most machines operate with a specific amount, based on size, of octyl alcohol, but there has been a tendency to add more in an effort to eliminate problems caused by poor maintenance. Generally, these efforts have been unsuccessful.
Suspended solids, which range from sub-micron particles to large chunks, contain the corrosion products, notably iron or copper oxides. The greater the chemical oxidation of the metallic portions of the absorption machine is, the higher the corrosion rate and the larger the amount of debris encountered will be.
The copper comes from the copper tubes, which dissolve in the lithium bromide. The resulting copper ions migrate to the more noble iron shell where the ions plate out of solution as pure copper metal. Chunks of copper fall off the shell wall and are transported by the solution. This plating action usually occurs when a free electrical charge is present to neutralize the positive charge of the copper ions. For example, pumps are a good source of electricity and finding the impellers coated with copper is common.
A mud-like deposit, which consists of many particles compacted into a sludge, is also quite common. This sludge, along with some larger chunks, is responsible for plugging heat exchangers and spray headers.
The specific gravity and corresponding percentage of solution reveal the concentration of the charge and can indicate machine problems. For example, a low specific gravity may indicate a water leak into the solution from a tube failure.
Specific gravity, considered in conjunction with corresponding temperature measurements, can also reveal whether a machine is operating properly. These readings are plotted on an equilibrium diagram to indicate the thermodynamics of the refrigeration cycle. The thermodynamic properties are then compared with design conditions to evaluate machine performance.
The pH and solution alkalinity provide similar information: pH is a rating scale indicating the relative acidity or alkalinity, while the solution alkalinity test measures the capacity of the solution to absorb excess acid or hydrogen ions to maintain solution intensity if a neutralizing agent is added to the lithium bromide. Lithium bromide solutions with similar pH values could have very different solution alkalinities.
Lithium bromide by itself is considered neutral—nonacid and non-alkaline; however, the solutions introduced into the absorption machine are made alkaline to help combat corrosion.
Air leaking into an absorber introduces oxygen and increases alkalinity. An analysis showing a higher than usual (or required) alkalinity could indicate an air leak. Ultimately, however, an air leak reduces solution alkalinity.
Solution alkalinity analysis is also critical for proper corrosion control. Some inhibitors require high alkalinities, while others need lower values, for proper control.
Dissolved copper and iron concentrations indicate levels of corrosion. A high dissolved-copper content reveals probable corrosion attack of the copper or copper-nickel tubing in an absorber. A high dissolved-iron value indicates attack of the shell.
Attack of the copper portions is more common than of the steel or ferrous areas. Concentrations of dissolved iron are usually much lower than those of dissolved copper because iron oxide does not usually stay dissolved in lithium bromide and readily precipitates out of solution.
Inhibitors are used to control corrosion and its aftereffects. The two most common inhibitors are lithium nitrate and lithium chromate.
If lithium nitrate is used, nitrate and ammonia must be monitored. The nitrate is chemically reduced by free hydrogen in solution. (The hydrogen is produced by the corrosive action of the charge on the mild steel components.) The ammonia thus formed increases the overall rate of copper corrosion and may cause stress—corrosion cracking.
Part of the ammonia can be removed through the purge system. But once ammonia is present, the potential for stress-corrosion failure will always exist. As little as 50 ppm of ammonia in lithium bromide can triple the corrosion rate of copper, and much less than that amount can, theoretically, induce stress corrosion. However, ammonia alone cannot cause stress-corrosion cracking, and absorbers may operate for years with ammonia and never experience stress corrosion.
Chromate corrosion inhibitors must be checked to see that the proper level and oxidation state are maintained. Too little chromate causes pitting, and too much adversely affects the solution’s chemistry.
The lithium bromide solution should be checked at least once a year, and whenever a problem occurs. More frequent analysis can indicate changes in the chemistry and alert the plant engineer to potential failures.
Typical Solution Analysis—Table II shows the results of a typical lithium bromide solution analysis. It reveals several important facts.
TABLE II. Typical Lithium Bromide Solution Analysis
|
Appearance: |
Black liquid; crystalline, clear after filtering |
|
Octyl Alcohol: |
Present, layer |
|
Suspended Solids: |
Slight; black appearance |
|
Specific Gravity: |
1.7323 |
|
Lithium Bromide Concentration: |
60.8 percent |
|
pH: |
11.29 |
|
Solution Alkalinity: |
0.162 milliequivalents/milliliter |
|
Dissolved Copper: |
186.2 ppm |
|
Dissolved Iron: |
7.6 ppm |
|
Lithium Nitrate Inhibitor Concentration: |
261.2 ppm |
|
Ammonia: |
35.8 ppm |
The visual appearance indicates corrosion: the clear lithium bromide solution is now a black liquid. Corrosion is confirmed by the dissolved iron content, which indicates probable shell corrosion. However, the suspended-solids reading indicates that the corrosion is probably not excessive. The pH reading is typical for lithium bromide, and the nitrate measurement indicates the inhibitor concentration.
The crystalline nature listed under "Appearance" relates to the sample, not to the entire lithium bromide charge inside the machine. Samples are usually taken while the machine is operating under vacuum. Removing the sample from a low-pressure environment and exposing it to atmospheric pressure alters its physical condition; in this case, crystals have formed.
Evaporator Water—Another absorption machine fluid requiring analysis is the evaporator water.
A high specific gravity indicates that the lithium bromide charge is leaking into areas of the absorber where it does not belong; the presence of ammonia in the evaporator water circuit signals a potential corrosion problem.
Richard Levine is a consultant dealing with Lithium Bromide-related problems in absorption refrigeration. He has 30 years experience in the field of Lithium Bromide analysis, interpretation, internal cleaning, and corrosion control. He can be reached at:
Richard Levine
LBD ASSOCIATES, LLC.
Randolph, NJ USA
973-895-5207
mailto:rslevine@lbdassociates.com
|
Click here to visit Richard S. Levine's entire Lithium Bromide website with more articles, features and technical information. |